Biologics have revolutionized the treatment of chronic inflammatory diseases such as psoriasis. These drugs are highly effective, but are only used to treat moderate-to-severe cases. In addition, though sold worldwide, they are costly for healthcare systems: in Belgium the prescription of biologics by dermatologists accounted for more than 51 million € in 2016. As biologics do not cure the disease and are required chronically, they pose a heavy burden on the healthcare budget. Remarkably though, effective guidelines in terms of dosing regimen, monitoring, and outcomes are lacking.
In the BIOLOPTIM project, we aim to develop algorithms for therapeutic drug monitoring (TDM) for biologicals used in the treatment of psoriasis: adalimumab (Humira®), secukinumab (Cosentyx®), ixekizumab (Taltz®), guselkumab (Tremfya®), risankizumab (Skyrizi®) and tildrakizumab (Ilumetri®). This includes measuring the drug ’s concentration in serum and how it benefits the patient (skin clearance). The development of evidence- based methods and guidelines in TDM will lead to improved treatment options for the patient and better quality of life, more cost-effective use of biologics, and reduced costs for our healthcare system.
This research was made possible through funding support of the Research Foundation–Flanders (FWO), Belgium, Grant number: T003218N. For any study related questions, don't hesitate to reach out to bioloptim@uzgent.be
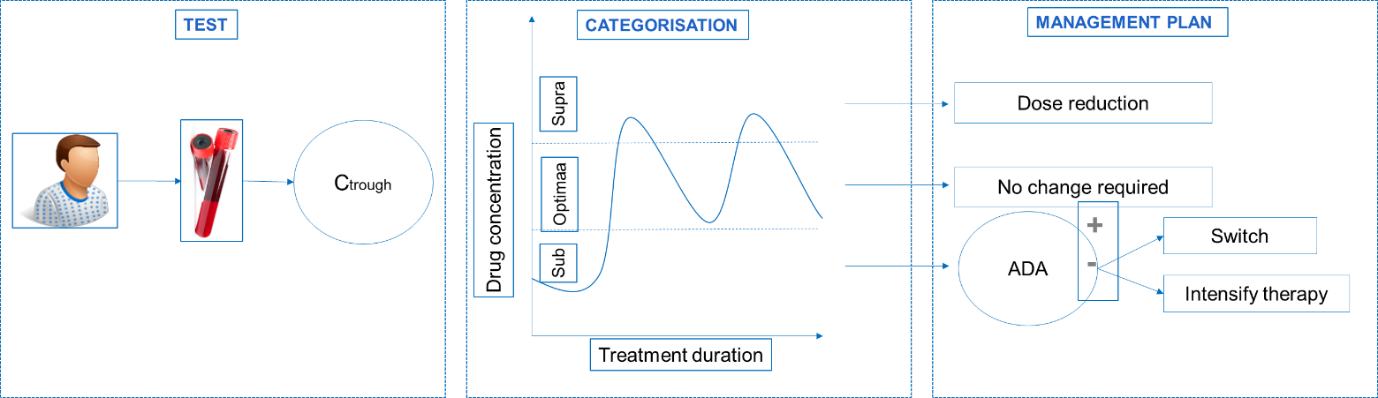
(Ctrough: the concentration reached by a drug immediately before the next dose is administered; ADA: Anti Drug Antibodies)
Made possible by:
